
#Depriester chart dew point how to
It is worth exploring these measures to better understand dew point and how to measure it relative to temperature, and humidity.ĭew point is essentially the temperature at which air saturates to form vapor, this is equal to 100% relative humidity.Ībsolute Humidity is a measure of water vapor per cubic meter of air.
Humidity Humidity is either measured in absolute terms, relative terms, or as specific humidity. Water vapor is a gas lighter than nitrogen or oxygen – the primary components of air. It is counter intuitive, since water is certainly heavier than air! But water in a gas form is not. So, when water vapor is in the air it displaces the air and lowers the overall molecular mass. The average molecular mass of air is around 29, and the molecular mass of water vapor is around 18. It is usually thought that air containing a higher humidity is denser than dry air, but this is not the case. Some areas are far drier than others, humidity can change depending on the time of day, and moisture in the air can be blown in or away by the wind. This can vary depending on various climate conditions. Humidity is also a measure of the amount of water vapor in the air. Of course, ice is water in its solid form. Similarly, changing states from water to ice is known as the frost point, as this is where frost would form. The temperature of the glass is colder than the air around the glass, and so, when the air, which contains water vapor makes contact with the glass, the vapor cools and begins to change state to become liquid. This happens because of a difference in relative temperature.
#Depriester chart dew point windows
Have you ever noticed your windows mist up on a freezing cold day? Or perhaps you have noticed droplets of water forming on the outside of an ice-cold glass? The higher the dew point, the more moisture there is in the air.
Water vapor is the gaseous state of water or water in a gas form. The measure marks the point to which the air must be cooled to reach a saturation point, as water vapor in the air condenses to form dew. This is probably because it is the most accurate reflection of what weather conditions feel like, as it combines temperature and humidity.ĭew point is the atmospheric temperature at which dew can form.

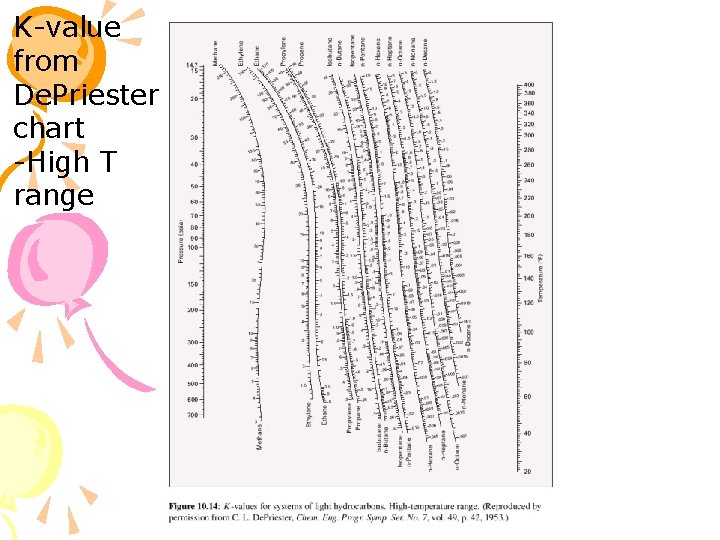
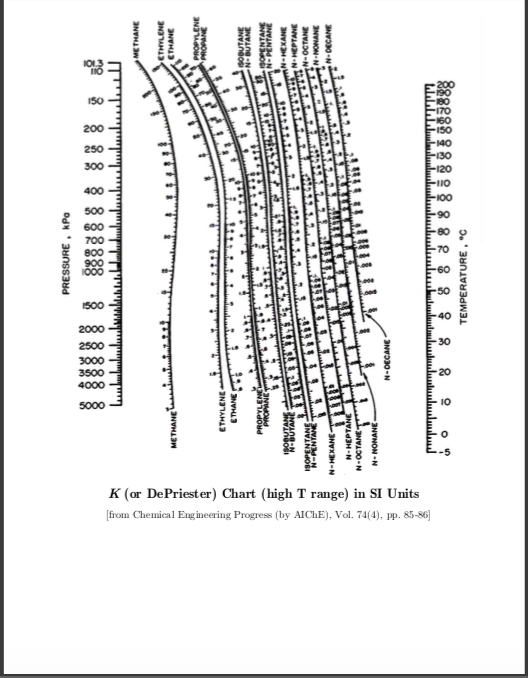
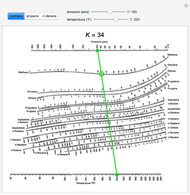
If K1 and K2 are functions of temperature and pressure only (ideal solutions), the flash curve can be calculated directly without iteration.Dew point is growing in its use by meteorologists as a measure of humidity. (13-2) reduces to the bubble-point equationįor a binary feed, specification of the flash-drum temperature and pressure fixes the equilibrium-phase concentrations, which are related to the K values by (13-12) to (13-14) are the bubble point, at which V = 0 and x, = zt, and the dew point, at which L = 0 and yt = zt, at which Eq. Alternatively, if T1 is fixed as mentioned earlier, a heat exchanger must be added before, after, or in place of the valve with the required heat duty being calculated from an energy balance. When converged, X Xt and X yt will each be very close to a value of 1, and, if desired, Ti can be computed from an energy balance around the valve if no heat exchanger is used. Generally, the iterations are continued until the calculated value of V/F equals to within ☐.0005 the value of V/F that was used to initiate that iteration. Otherwise, the Ki values must be periodically updated for composition effects, perhaps after each iteration, using prorated values of x, and y, from Eqs. Then the resulting calculations are straightforward. Values of K are constants if they are independent of liquid and vapor compositions. Procedures such as the Newton-Raphson, secant, false-position, or bisection method can be used to solve Eq. Any one of a number of numerical root-finding FIG. (13-12) and (13-13) and L from the total mole balance. The component mole balance (Fz, = Vy, + Lx,), phasedistribution relation (K, = y,/xi), and total mole balance (F = V + L) can be combined to giveĮquation (13-14) is solved iteratively for V/F, followed by the calculation of values of x, and y, from Eqs. A popular method is the following due to Rachford and Rice. Except for an ideal binary mixture, procedures for calculating an isothermal flash are iterative. The calculation for a point on the flash curve that is intermediate between the bubble point and the dew point is referred to as an isothermal-flash calculation because T2 is specified.


 0 kommentar(er)
0 kommentar(er)
